|
||||||||||||||||
|
||||||||||||||||
%
responses 2007 ('03) |
||||||||||||||||
y4 |
y8 |
|||||||||||||||
| 1. What does a candle need to keep burning? |
||||||||||||||||
oxygen |
6 (9) |
32 (21) |
||||||||||||||
air |
7 (13) |
13 (16) |
||||||||||||||
| In this activity, I’m going to light the candle in this glass. Then I’m going to pour some vinegar onto the baking soda at the bottom. 2. Before we do this I want you to tell me what you think will happen to the baking soda when I add the vinegar. |
||||||||||||||||
| Baking soda will react with vinegar: | yes, using word |
1 (0) |
6 (5) |
|||||||||||||
yes, more general |
1 (2) |
3 (4) |
||||||||||||||
baking soda will fizz/make bubbles/ give off a gas |
40 (31) |
73 (69) |
||||||||||||||
baking soda will give off carbon dioxide |
0 (1) |
2 (3) |
||||||||||||||
| I’m going to light the candle now. Light the candle. Now, I’m going to squirt the vinegar down the side of the glass onto the baking soda so that it becomes very wet. Squirt vinegar down side of glass onto baking soda. 3. What happened to the baking soda? |
||||||||||||||||
baking soda reacted with vinegar |
1 (3) |
10 (6) |
||||||||||||||
baking soda fizzed/made bubbles/ gave off a gas/frothed/foamed |
65 (67) |
80 (77) |
||||||||||||||
baking soda gave off carbon dioxide |
1 (0) |
2 (3) |
||||||||||||||
| 4. What happened to the candle flame? | not marked |
• |
• |
|||||||||||||
| 5. What do you think might have put out the candle flame? |
||||||||||||||||
carbon dioxide (from the reaction) |
1 (2) |
6 (6) |
||||||||||||||
gas/fumes (from the reaction) |
1 (3) |
16 (15) |
||||||||||||||
| 6. Do you know any gases that would put out a candle flame? |
||||||||||||||||
carbon dioxide |
2 (3) |
17 (18) |
||||||||||||||
other gases that do not support
combustion (e.g. nitrogen, helium, neon, argon) |
1 (1) |
4 (3) |
||||||||||||||
Total
score: |
4–13 |
4 (2) |
35 (29) |
|||||||||||||
3 |
5 (13) |
17 (17) |
||||||||||||||
2 |
30 (26) |
27 (25) |
||||||||||||||
1 |
37 (38) |
16 (22) |
||||||||||||||
0 |
24 (21) |
5 (7) |
||||||||||||||
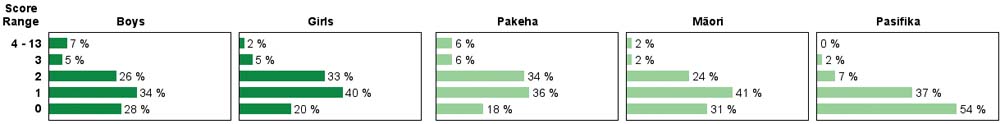
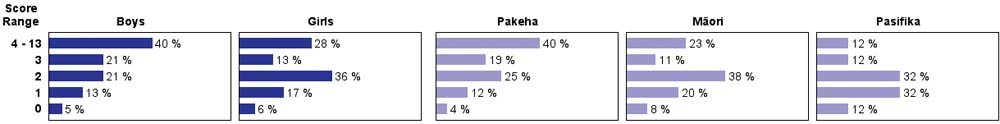
| Subgroup Analysis [Click on charts to enlarge] : |
| Commentary: |
| Good performance on this task required chemical knowledge, careful observation and interpretation. About 60% of year 4 students, compared to 20% of year 8 students, had very little success with this task. There was little change in performance at either year level between 2003 and 2007. Year 8 boys scored higher than year 8 girls while most Pasifika students had low scores at both year levels. |